
By Mindi Dearing, Compliance Manager
Beaver Water District’s Mission is “To sustainably provide our customers with safe, economical drinking water”. In order to do this, we must continuously comply with the standards that the U.S. Environmental Protection Agency (EPA) sets for drinking water to protect against naturally occurring and man-made contaminants. Maximum contaminant levels for the standards are set by the EPA and are enforced by the Arkansas Department of Health in the state of Arkansas.
To aid in your understanding, Beaver Water District (BWD) prepares an annual report that includes a variety of analytical results from the previous year (in this case 2023). The analysis is conducted by the Arkansas Department of Health Laboratory and the professionally trained staff in BWD’s accredited laboratory.
In addition to the Annual Water Quality Report, a section containing data for the most commonly requested parameters titled “Finished Water Quality Update”, as seen below, is posted monthly on our website.
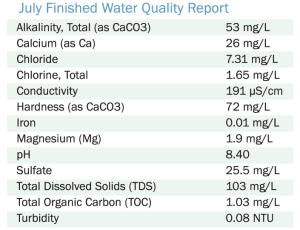
Below are some descriptions (and their importance) for some of the commonly tested water quality parameters:
pH
pH is the measurement of how acidic or basic the water is. The pH scale is from 0 to 14. A pH of less than 7.0 means the water is acidic while a pH of greater than 7.0 is basic. A pH of 7.0 is considered neutral.
Hardness and Alkalinity
Hardness in water is caused by the presence of calcium and magnesium ions. Hard water can cause an increase in soap and detergent usage and increase mineral deposits (scaling) in hot water tanks and plumbing systems. The light-colored ring that forms in a pan after boiling water is a result of the hardness of the water. Soft water doesn’t require as much soap to work up a lather; however, if the water is too soft you will have trouble rinsing the soap off and may be left with a slimy feeling. The 2023 average hardness of the water distributed by Beaver Water District was 74 parts per million (ppm) which is moderately hard according to the table below.

Alkalinity is the quantitative capacity of water to neutralize an acid; that is, the measure of how much acid can be added to a liquid without causing a significant pH change. Automatic dishwasher detergents, most cleansers and hard surface cleaners utilize alkalinity for their cleaning ability. Hardness and alkalinity are often talked about together because many chemical substances contribute to both hardness and alkalinity.
Coliform
Coliforms are a group of non-pathogenic bacteria that are naturally present in the environment as well as in feces. Total Coliforms are not a health threat in itself but are used to indicate if other potentially harmful bacteria may be present.
Turbidity
Soil runoff is one of the main sources of turbidity. Turbidity is a measure of the cloudiness of water. It is used to indicate water quality and filtration effectiveness (e.g., whether disease-causing organisms are present). Higher turbidity levels are often associated with higher levels of disease-causing microorganisms such as viruses, parasites, and some bacteria.
Trihalomethanes and Haloacetic Acids
Disinfection byproducts are compounds that are formed when disinfectants like chlorine combine with organic matter in the water. Trihalomethanes (THMs) and Haloacetic Acids (HAA5) are two classes of these byproducts that are regulated because of potential negative health effects and have limits established by the Environmental Protection Agency (EPA) of 80 ppb and 60 ppb respectively.
Total Organic Carbon (TOC)
Total Organic Carbon (TOC) is a measure of the organic content in the water. Since the disinfection byproducts (DBP) listed above are formed from the combination of chlorine and organic matter, TOC is used as a “precursor” for DBP formation. TOC samples can be analyzed more quickly and are less costly to run than THM and HAAs.
Subscribe now to stay informed with monthly email updates on water quality results.
